In chemistry, it sometimes difficult for some student to distinguish or grasp the full understanding between a Base and an Alkali. Sometimes, you'll probably hear something like "all alkalies are bases but not all bases are alkalies". Without much more in depth reasoning, you won't get it right. This post will save you that stress. Let start by defining a base.
What is really a base
Substances that have a soapy feel, taste bitter, turns red litmus paper blue, and generally possesses the ability to neutralize an acid are classified as bases.
A Base is simply a substance that is used to neutralize an acid. But how? To neutralize simply means to weaken the strength of something by adding an opposing effect. So in this case, neutralization reactions are when an acid react with a base to form water and a salt. So a base react with an acid in order to make it neutral. One reason an acid is neutralized is in the sector of agriculture, where a soil happens to be more acidic and it pH drops. By adding a substance(like a base) which will neutralize the acidity of the soil, it will be possible to raise the pH to more neutral levels for plant to grow effectively.
Bases comes in form of metal hydroxides (metal+ OH-) and metal oxides ( metal+ O2-). Examples of bases include: Sodium Oxide(Na2O), Copper Oxide (CuO), potassium Oxide (KOH), Sodium hydroxide (NaOH), calcium hydroxide (Ca(OH)2 ) Calcium Oxide (CaO), potassium Oxide (K2O) etc.
Then what is an alkali
You may have notice in the above examples that some bases ends with "OH", while some are accompanied by just a single "O". That is the point. Those that ends with OH are example of bases called alkali.
An Alkali is just a kind of base that dissolves in water. Let use our above examples again.

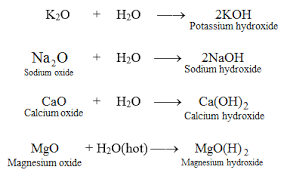
















0 comments:
Post a Comment