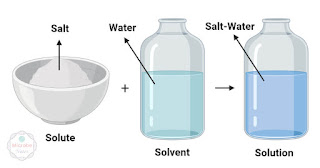
Solution: A homogeneous mixture, which may be liquid, gas or solid, formed by dissolving one or more substances.
2. It is composed of just a single phase. Hence, it impossible to distinguish one components from another. For example, In a perfect sugar solution, you cannot distinguish the sugar in the water.
3. The solutes in a solutions cannot be seen by the naked eye.
4. Beam of light passing through it doesn't scatter, because it components are very tiny(about 1nm in diameter)
5. Component making up a solution cannot be separated by ordinary filtration.
- Mixture of salt or sugar in water
- Metallic alloys e.g steel, bronze are examples of solid solutions.
- Air
- Tea/coffee
- Bleach etc
- Juice
Characteristics of a solvent
- Water (universal solvent)
- Alcohols like methanol, ethanol etc.
- Petroleum products and hydrocarbons like benzene, glycerol, acetone. toluene
- Chloroforms
- Milk, Oil, citric acids etc.
Can a solvent be in a Solid form?
When most people think of the term solvent, a liquid medium always comes to mind; however, there are both cases in which a solid or a gas can also acts as solvents.
While it is true in some cases that when dissolving, the solvent has to be in it liquid state, this is because the solvent has to get "between" the molecules of the solute to do its job, and that can only work when the solvent is liquid. This explanation thus appears not to be always the case. Lets see some examples:
— Air is a homogeneous mixture of different gases including oxygen, nitrogen, and carbon(iv)oxide. This makes air a solution. The components of air making it a solution consists of different gases both acting as a solute and as a solvents.
— Steel and bronze are also an example of a homogeneous mixtures consisting of various solid metals. Steel consist of a mixture of iron and carbon, while bronze is composed of copper and tin. Here we can see that two solids also acts as a solute and a solvents, but in this case, both the solid-solute and the solid-solvents has to be melted before the can be dissolved.
Solute: Any substance that is dissolved in a solvent to create a solution is called a solute.This means that in a solution, the solute is the substance that is being dissolved by a solvent. For a solute to be dissolved by a solvents, the attractive force between their them must be stronger enough to overcome the molecular forces holding their molecules. This will make them uniformly mix together.
The solutes only takes the smaller portion in a solution, while the solvents will take a larger portion.
A solutes occurs mostly as solids, but they can also be in liquids and gaseous states.
Examples of solutes
- Sugar in a coffee
- Salts in seawater
- Oxygen in air
- Zinc, tin, and carbon in brass, bronze, and steel respectively.
- CO2 in carbonated beverages like coca cola.
Characteristics of a solute
1. They possess the smallest portion in a solution
2. They can be in solid, liquid or gaseous state
3. They have a higher boiling points than solvents.
4. They possesses a solubility limits and they can be saturated.
EXTRA TERMS.
Solubility
It is the ability of a substance to dissolve in another substance, especially water. be it solid, liquid or gas. Different kinds of solutes and solvents has their different degree of how they dissolves or are being dissolved in a solution. And this depends on a number of factors, including temperature, pressure and mass.
Solubility Limit
This is the limit to how much a solute can be dissolved by a solvents in a solution. Using a salt and water example, if you keep adding excess salt to water, you will finally reach a point where the added salt remains as granuals and does not dissolve. At that point, the “solubility limit” has been reached. The solution is “saturated.” Once the solvent has dissolved all of a material that it can, it is no longer a solvent for that material. The solubility limit, though, typically increases as temperature increases. Increasing the temperature of a salt/water solution will allow more salt to be dissolved by raising the saturation limit. However, even a saturated solution may still be a solvent for another material.
SUMMARY
A solution is a homogeneous mixture of two or more substances.
A solution consist of a solute and a solvents
The substance which is being dissolved is called a solute and the substance in which the solute is dissolved is called a solvent. A mixture of solute and solvent is called a solution.


















0 comments:
Post a Comment