The separation of electric charges into positive and negative still sounds much of a brain box to some curious new physicist, especially in the field of electrical and electronics engineering. But the interesting fact about this is that it was all because of Benjamin franklin's arbitrary decision.
Are you actually a curious type? Do you want to know they full fact behind the ideas that :
- Electrons possesses a negative charge, while protons are positively charged.
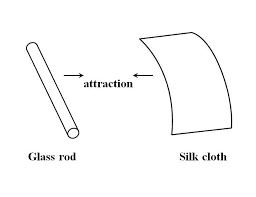
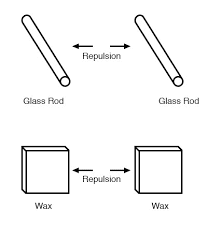
- Like charges repel while unlike charges attract
What are charges anyway
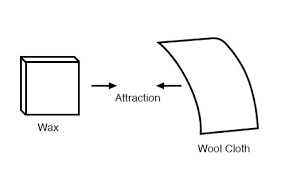
Following Franklin’s speculation of the wool rubbing something off of the wax, the type of charge that was associated with rubbed wax became known as ”NEGATIVE” (because it was supposed to have a deficiency of fluid) while the type of charge associated with the rubbing wool became known as ”POSITIVE” (because it was supposed to have an excess of fluid). Little did he know that his innocent conjecture would cause much confusion for students of electricity in the future!
However, electrons have significantly more freedom to move around in an atom than either protons or neutrons. In fact, they can be knocked out of their respective positions (even leaving the atom entirely!). If this happens, an important imbalance will occur. Electrons and protons are unique in the fact that they are attracted to one another over a distance. It is this attraction over distance which causes the attraction between rubbed objects, where electrons are moved away from their original atoms to reside around atoms of another object.
Electrons tend to repel other electrons over a distance, as do protons with other protons. The only reason protons bind together in the nucleus of an atom is because of a much stronger force called the strong nuclear force which has effect only under very short distances. Because of this attraction/repulsion behavior between individual particles, electrons and protons are said to have opposite electric charges. That is, each electron has a negative charge, and each proton a positive charge.
The process of electrons arriving or leaving is exactly what happens when certain combinations of materials are rubbed together: electrons from the atoms of one material are forced by the rubbing to leave their respective atoms and transfer over to the atoms of the other material. In other words, electrons comprise the ”fluid” hypothesized by Benjamin Franklin. The result of an imbalance of this ”fluid” (electrons) between objects is called static electricity. It is called ”static” because the displaced electrons tend to remain stationary after being moved from one insulating material to another. In the case of wax and wool, it was determined through further experimentation that electrons in the wool actually transferred to the atoms in the wax, which is exactly opposite of Franklin’s conjecture! In honor of Franklin’s designation of the wax’s charge being ”negative” and the wool’s charge being ”positive,” electrons are said to have a ”negative” charging influence. Thus, an object whose atoms have received a surplus of electrons is said to be negatively charged, while an object whose atoms are lacking electrons is said to be positively charged, as confusing as these designations may seem. By the time the true nature of electric ”fluid” was discovered, Franklin’s nomenclature of electric charge was too well established to be easily changed, and so it remains to this day.




















0 comments:
Post a Comment